Introduction
Imagine microscopic, self-assembled robots crafted from human cells, meticulously engineered to navigate the body and repair tissues at a cellular level. This visionary concept is now a reality with Anthrobots—dynamic biological machines constructed from adult human lung cells. These biobots demonstrate the incredible plasticity of human cells, reshaping our understanding of cellular function. By leveraging advanced bioengineering techniques, scientists have created structures capable of self-assembly, motility, and even tissue repair, offering transformative possibilities in medicine. From targeted therapies to regenerative breakthroughs, Anthrobots could revolutionize healthcare.
From Airway Cells to Self-Propelled Spheres
The journey of Anthrobots begins with adult human bronchial epithelial (NHBE) cells—cells traditionally forming flat airway linings. By culturing these cells in three-dimensional environments and releasing them into a low-viscosity medium, researchers induced a remarkable transformation. These cells self-assembled into ciliated spheroids, flipping their polarity to reorient cilia outward. This reconfiguration enabled the spheroids to propel themselves autonomously, an extraordinary demonstration of cellular plasticity. Unlike traditional bioengineering, this process required no genetic modification or external scaffolding, showcasing the latent potential of human cells to construct functional, motile structures.
A Spectrum of Movement and Morphology
Anthrobots are not uniform in their behavior. They exhibit distinct motility patterns—some moving in tight circles, others along straight paths, and a third group adopting curvilinear trajectories that blend circular and linear motion. This behavioral diversity corresponds to specific morphological traits. Smaller, spherical Anthrobots predominantly exhibit circular motion, while larger, irregularly shaped bots are inclined to move linearly. Curvilinear Anthrobots combine traits of both. These insights, derived from extensive timelapse video analyses, reveal that Anthrobots are not random assemblages but intricately organized systems with clear correlations between their structure and function.
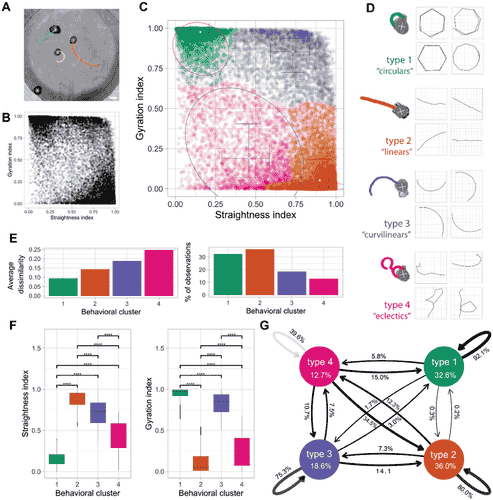
Bilateral Symmetry: A Universal Blueprint
Remarkably, Anthrobots display bilateral symmetry, a characteristic integral to many living organisms. Linearly moving Anthrobots, in particular, exhibit pronounced symmetry along their movement axis. This finding highlights parallels between these synthetic lifeforms and natural developmental principles shaping animal bodies. Detailed analyses of cilia distribution patterns confirmed this symmetry, further emphasizing the intricate self-organization of Anthrobots. Such observations suggest that these biobots may offer insights into fundamental biological processes while serving as a platform for advanced bioengineering.
Facilitating Repair in Damaged Neuronal Tissue
Beyond their inherent motility, Anthrobots have demonstrated remarkable therapeutic potential. When introduced to neuronal cell sheets with induced scratches, Anthrobots actively traversed these gaps, promoting the regrowth of neurons. This healing capability was absent in control setups with passive materials, underscoring the active role of Anthrobots in tissue repair.
Researchers took this a step further by creating “superbots” through the fusion of multiple Anthrobots. These larger constructs acted as living bridges over neuronal gaps, accelerating robust tissue regeneration beneath them. The implications are profound, offering a glimpse into future applications of Anthrobots as vehicles for delivering therapeutic agents or stimulating localized regeneration in damaged tissues.
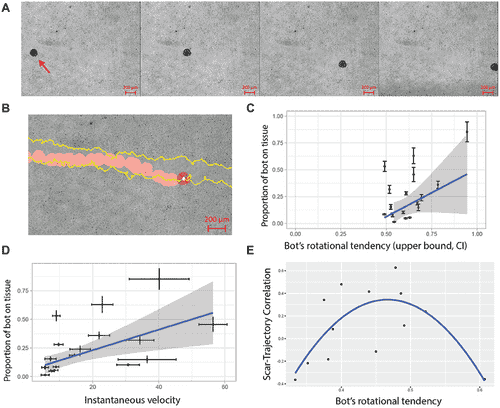
Expanding the Frontiers of Biomedicine
The study of Anthrobots raises critical questions about the potential of adult human cells to self-organize into functional, motile systems. This work represents a significant step in understanding the breadth of morphological and behavioral possibilities achievable with wild-type genomes, free from genetic manipulation or external fabrication.
The capacity of Anthrobots to traverse and repair damaged tissues, combined with their ability to self-assemble and exhibit diverse motility patterns, positions them as powerful tools for regenerative medicine. Future research could explore enhancing their functionality through synthetic biology, potentially creating biobots tailored for specific therapeutic purposes.
Conclusion
Anthrobots exemplify the untapped potential of human cells to self-construct into sophisticated, functional entities. From their beginnings as airway epithelial cells to their transformation into motile, tissue-repairing biobots, they represent a groundbreaking intersection of biology and engineering.
This research not only advances our understanding of cellular plasticity but also opens doors to new medical paradigms. With continued exploration, Anthrobots could lead to innovations in bio-robotics, regenerative therapies, and beyond, paving the way for a future where our cells serve as autonomous agents of healing and discovery.
Reference
Gumuskaya, Gizem, Pranjal Srivastava, Ben G. Cooper, Hannah Lesser, Ben Semegran, Simon Garnier, and Michael Levin. “Motile Living Biobots Self-Construct from Adult Human Somatic Progenitor Seed Cells.” Advanced Science 11, no. 11 (2024): 2303575. https://doi.org/10.1002/advs.202303575.
Let us know your thoughts! Sign up for a Mindplex account now, join our Telegram, or follow us on Twitter.

.png)

.png)


.png)
