How Memory Stays Sharp: The Role of KIBRA and PKMζ in Long-Term Memory Maintenance
Dec. 02, 2024. 5 mins. read.
15 Interactions
A molecular duo, KIBRA and PKMζ, may hold the secret to long-lasting memories. Learn how their interaction could revolutionize memory-related treatments.
Introduction
Why do some memories linger for a lifetime while others vanish soon after forming? Neuroscientists have long grappled with this puzzle, especially given the brain’s constant renewal of its molecular components. A recent breakthrough study has illuminated a key aspect of this phenomenon: the interaction between two proteins, KIBRA and PKMζ, provides a scaffolding that stabilizes memory. This remarkable discovery not only explains how long-term memory persists but also opens potential avenues for treating memory-related disorders like Alzheimer’s disease.
The Challenge of Long-Term Memory Retention
Memory is stored in the brain through the strengthening of synaptic connections, a process known as long-term potentiation (LTP). However, the machinery that sustains these connections, from proteins to receptors, is continually replaced. This turnover creates a paradox: how can memories persist for decades if the molecular components of the brain are so short-lived? Decades ago, Francis Crick suggested that stable interactions between molecules might hold the key to this mystery. Building on this idea, researchers focused on KIBRA, a scaffolding protein tied to memory performance, and PKMζ, a kinase known for its role in sustaining synaptic changes.
KIBRA and PKMζ: The Anchors of Memory
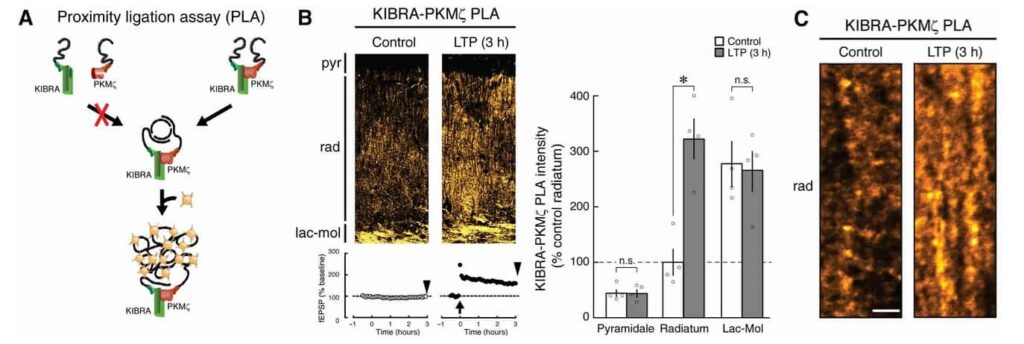
KIBRA and PKMζ work together to maintain the stability of synaptic connections crucial for memory. PKMζ, an atypical kinase, is persistently active, bypassing the need for external activation, and plays a vital role in reinforcing the connections at memory-encoded synapses. KIBRA serves as a docking station for PKMζ, anchoring it at synapses to ensure that the structural changes necessary for memory endure. Without KIBRA’s anchoring action, PKMζ cannot localize effectively, causing the connections to weaken and memories to fade.
Experiments highlighted how synaptic activity intensifies the interaction between KIBRA and PKMζ. Using cutting-edge proximity ligation assays, researchers observed that strong neuronal stimulation led to the accumulation of KIBRA-PKMζ complexes at active synapses. These complexes were particularly concentrated in regions of the brain linked to learning and memory, such as the hippocampus.
Testing the Limits of the KIBRA-PKMζ Partnership
The study explored deeper by introducing antagonists that blocked the interaction between KIBRA and PKMζ. The consequences were striking: blocking this interaction disrupted established LTP while leaving basal synaptic transmission unaffected. This finding underlined the selective importance of the KIBRA-PKMζ complex in maintaining memory-associated synaptic changes.
To explore how this disruption impacted behavior, the researchers tested mice trained in spatial memory tasks. Mice conditioned to avoid a specific area of their environment lost this memory after the introduction of an inhibitor that blocked KIBRA-PKMζ coupling. However, their ability to learn and form new memories remained intact. This specificity highlights the unique role of KIBRA and PKMζ in stabilizing existing memories, distinguishing it from the processes involved in learning.
A Molecular Mechanism That Defies Turnover
One of the study’s most intriguing findings was how the KIBRA-PKMζ interaction enables memory to persist despite the turnover of individual molecules. The research confirmed that while the proteins themselves are replaced within hours or days, the interaction between them is continuously re-established at memory-relevant synapses. This dynamic process ensures that the “scaffold” supporting memory remains intact even as its components are renewed.
The researchers proposed a model of “persistent synaptic tagging,” where KIBRA functions as a tag that attracts newly synthesized PKMζ molecules to the right locations. This process sustains the structural integrity of memory-encoded synapses, making it possible for memories to outlast the lifespan of their molecular building blocks.
Implications for Memory Disorders and Treatment
The discovery of the KIBRA-PKMζ interaction has profound implications for understanding and treating memory-related conditions. If this partnership is essential for maintaining memory, then therapies aimed at enhancing or mimicking this interaction could potentially combat age-related memory decline or neurodegenerative diseases like Alzheimer’s.
One promising avenue involves developing drugs that stabilize or amplify the KIBRA-PKMζ connection, strengthening synaptic changes and improving memory retention. Conversely, selectively disrupting this interaction could help erase traumatic memories, offering hope for conditions such as post-traumatic stress disorder (PTSD). The study also raises the possibility of targeted therapies that activate PKMζ in specific brain regions, enhancing memory resilience in the early stages of dementia.
A Glimpse Into the Future of Memory Research
The partnership between KIBRA and PKMζ represents a significant leap forward in our understanding of how the brain preserves memories over time. By acting as a molecular scaffold, these proteins ensure the stability of synaptic changes that underpin long-term memory. This discovery not only sheds light on the biological basis of memory but also offers a blueprint for future research and therapeutic innovation.
As scientists continue to unravel the intricacies of this molecular duo, the potential for groundbreaking treatments becomes clearer. From enhancing memory in aging populations to mitigating the effects of neurodegenerative diseases, the KIBRA-PKMζ connection offers a promising pathway for addressing some of the most pressing challenges in neuroscience.
Conclusion
Memory, once thought to be an ephemeral and mysterious phenomenon, is now being understood at the molecular level. The discovery of the KIBRA-PKMζ partnership provides a compelling explanation for how the brain defies the constraints of molecular turnover to preserve memory. This research not only advances our understanding of the brain’s inner workings but also points toward a future where memory loss is no longer an inevitable consequence of aging or disease.
Through continued exploration of this groundbreaking mechanism, we inch closer to unlocking the full potential of memory science and delivering tangible benefits to those affected by its decline. The intricate dance between KIBRA and PKMζ reminds us that even the smallest molecular interactions can have profound implications for the human experience.
Reference
Tsokas, Panayiotis, Changchi Hsieh, Rafael E. Flores-Obando, Matteo Bernabo, Andrew Tcherepanov, A. Iván Hernández, Christian Thomas, et al. “KIBRA Anchoring the Action of PKMζ Maintains the Persistence of Memory.” Science Advances 10, no. 26 (June 26, 2024). https://doi.org/10.1126/sciadv.adl0030.
Let us know your thoughts! Sign up for a Mindplex account now, join our Telegram, or follow us on Twitter.

.png)

.png)


.png)








5 Comments
5 thoughts on “How Memory Stays Sharp: The Role of KIBRA and PKMζ in Long-Term Memory Maintenance”
Thanks for this summary of a very deep study report on memory chemistry. I had knowledge once of an experience on a locked ward in a nursing home - many dementia/alztheimers patients. Early generational music was played to the residents - 1920s-1950s music. Anecdotal reports indicated many of the patients memories re-emerged while the music played. I wonder if there was a connection on a chemical level that increased or enhanced the long term memory?
🟨 😴 😡 ❌ 🤮 💩
This breakthrough could revolutionize memory-related treatments for Alzheimer’s and PTSD. Hope on the horizon
🟨 😴 😡 ❌ 🤮 💩
The discovery of how KIBRA and PKMζ work together to keep memories intact is truly fascinating. It's exciting to think that this could lead to breakthroughs in treating memory loss and diseases like Alzheimer's. It’s amazing how something so small could have such a big impact on our understanding of memory.
🟨 😴 😡 ❌ 🤮 💩
This is a fascinating discovery! The KIBRA-PKMζ partnership opens up exciting new possibilities for understanding and potentially treating memory disorders, offering real hope for the future.
🟨 😴 😡 ❌ 🤮 💩
"KIBRA and PKMζ are like the molecular DJ duo keeping our memories groovy on the brain's dance floor! This article brilliantly showcases how these two proteins tag-team to make sure our synaptic connections don't miss a beat. Whether it's saving precious moments or finding new treatments, their work is the ultimate playlist for long-term memory. Fascinating stuff! 🎵🧠"
🟨 😴 😡 ❌ 🤮 💩